
History
An Italian success

Eurospital’s journey is full of challenges and product and market affirmations in the pharmaceutical and diagnostic industry that continues to this day, day after day.
Founded in Trieste in 1948, Eurospital is an Italian company that has maintained its original identity over time, thanks to the managerial continuity of the Kropf family, which has led the company for three generations.
Today, at the age of 75, Eurospital continues to look to the future and to seek those “innovations for a better life” that are the expression of the philosophy of corporate action.

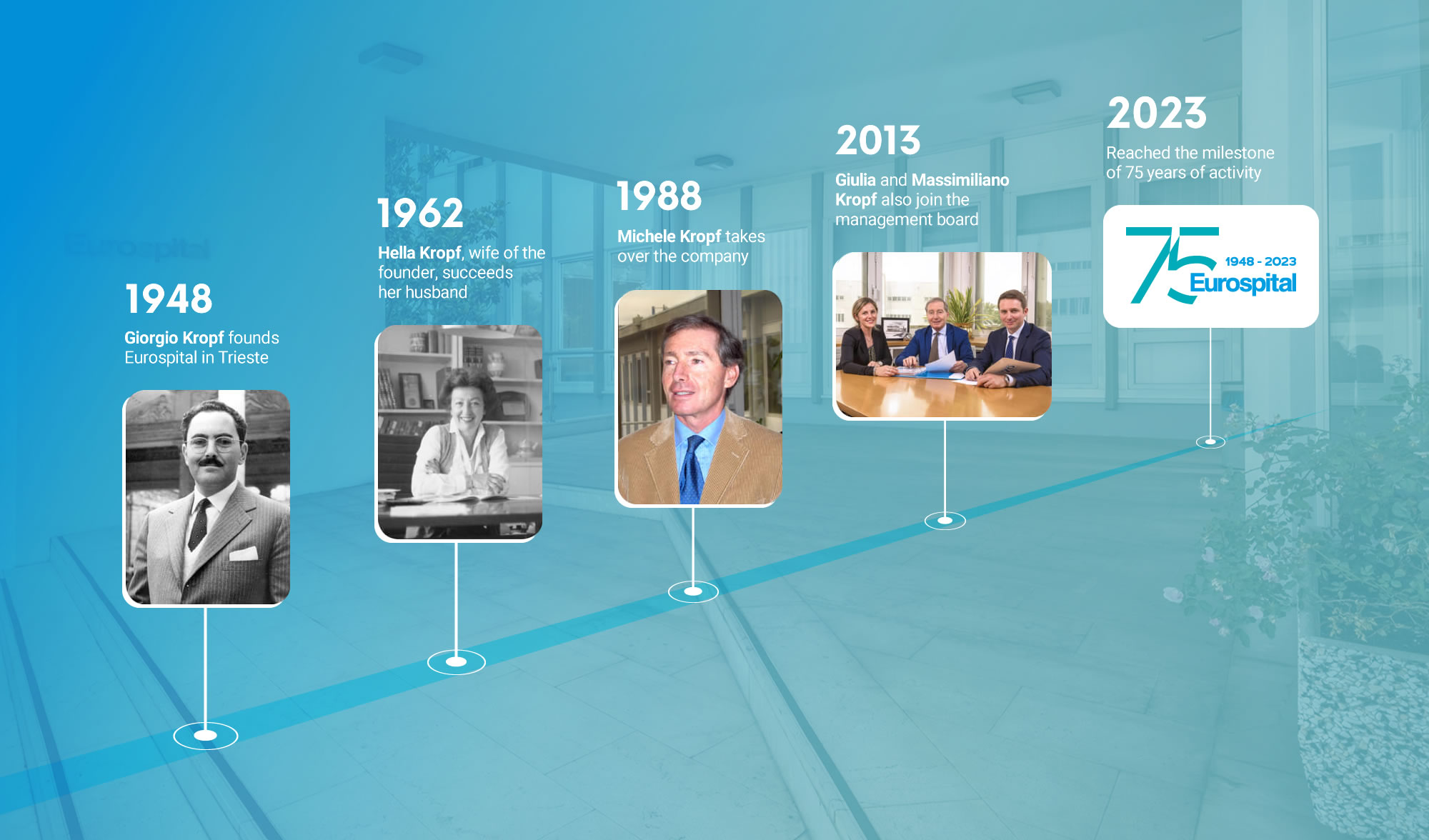
1948
Giorgio Kropf founds Eurospital in Trieste

1962
Hella Kropf, wife of the founder, succeeds her husband

1988
Michele Kropf, takes over the company

2013
Giulia and Massimiliano Kropf also join the management board

2023
Reached the milestone of 75 years of activity
1948
Giorgio Kropf, together with a pharmacist he knew, founds Farmimport in Trieste, the original nucleus from which Eurospital will develop.
First historical step: Streptomycin is imported from the American company Merck. The first antibiotic in Italy to be used successfully in the treatment of tuberculosis.1950
Giorgio Kropf collaborates, together with the colossus Don Baxter, in the construction of the first Blood Bank of Trieste, the second to be established in Italy after Turin.
The line of infusion solutions that will establish itself as one of the company's flagship products is imported from Baxter.1952
The production laboratories are built and an agreement is also reached with the London-based Allen & Hanburys to produce directly in Trieste the infusion solutions and other licensed products of these two pharmaceutical giants.1955
Production begins under license of Haliborange, effective multivitamin, one of the most appreciated and still most successful medicinal specialties of Eurospital's pharmaceutical sector.1958
The BAXTER line of intravenous solutions and blood equipment is complemented by a line of phrycohematheques and medical-hospital equipment.
At the same time, the marketing of Dequadin Tintura 28 ml and 10 ml, a drug for the treatment of inflammation of the oral cavity, begins.1962
Hella Kropf, great innovator and life partner of Giorgio Kropf, suddenly passed away, succeeds her husband, becoming one of the first Italian women to lead a company.1967
The company moves permanently to the new – current – headquarters in via Flavia.1968
Stylex, the first single-use syringe, is manufactured and marketed. An important step forward in the fight against hospital infections.1970
Significant productive and commercial development, doubling the number of employees.1971
Hella Kropf founded – as President – the Delegation of the Three Venetians of the A.I.D.D.A. (Association of Women Entrepreneurs and Business Executives), affiliated to the F.C.E. (Les Femmes Chefs d 'Entreprises Mondiales).1979
Michele Kropf, son of Giorgio and Hella, enters the company inaugurating a new course. The positioning of the company in the pharmaceutical and hospital sector is consolidated.1984
The company name becomes Eurospital Pharma.1986
The Research and Development facility is born and assumes a central role in the diagnostic field. The aim is to introduce, in Italy and abroad, innovative products under the Eurospital brand.
On 20/11/1986 the first diagnostic kit entirely produced by EUROSPITAL was released. It’s the first of many others. .1988
The company management passes to Dr. Michele Kropf and the company is reorganized into three divisions: Pharmacies, Diagnostics and Hospital.1989
With Burlo Garofalo and the Universities of Naples and Trieste, αGliatest IgA is developed and produced. It’s the first and innovative in vitro test to detect antibodies against gluten, responsible for the manifestation of Celiac disease.1990
The Sterilens line is launched, the first range of contact lens solutions in pharmacies, and the brands for Eurospital infusion solutions, Haliborange, Dequadin, Torch and Non Ad Garze are acquired in ownership.
The Hospital Division develops Euroset, the first double-graded flow regulator for the controlled administration of medicine. The device is introduced into hospitals and health centers.1995
First Italian company in the sector to certify its Quality System for the production of in vitro diagnostics according to ISO 9001:1994.1997
Acquisition of the exclusive license to use the worldwide patent for the use of tissue transglutaminase as a specific marker for the diagnosis of celiac disease, thus being able to develop the innovative Eu-tTG IgA test.2000
The Eurospital laboratories develop Calprest, an in vitro test for the determination of the fecal concentration of calprotectin, which has become, within fifteen years, an international reference point for the non-invasive verification of inflammatory states in the intestine.2008
From the twenty-year commitment in the study and diagnosis of celiac disease, a food brand is born expressly designed for people with celiac disease: Piaceri Mediterranei.2013
Eurospital's leadership directly involves the third generation, Massimiliano and Giulia Kropf, sons of Michele and grandchildren of the founder Giorgio.2017
First company to receive FDA certification for the Calprotectin test for the diagnosis of Chronic Inflammatory Bowel Diseases (ICD).
Eurospital obtains, as the first company in the sector in Italy, the certification from TÜV America of the necessary requirements for the United States and Canada under the MDSAP program.2018
Eurospital celebrates its 70th anniversary.
Watch the video on Youtube2020-21
Constant commitment by Eurospital to support Italian laboratories and pharmacies in the fight against SarsCov2 with innovative and high quality products.2022
Eurospital is among the first Italian companies to have received the IVDR Certificate for in vitro diagnostic medical devices from the notified body TÜV Süd, one of the largest international providers of certification and approval of medical devices.